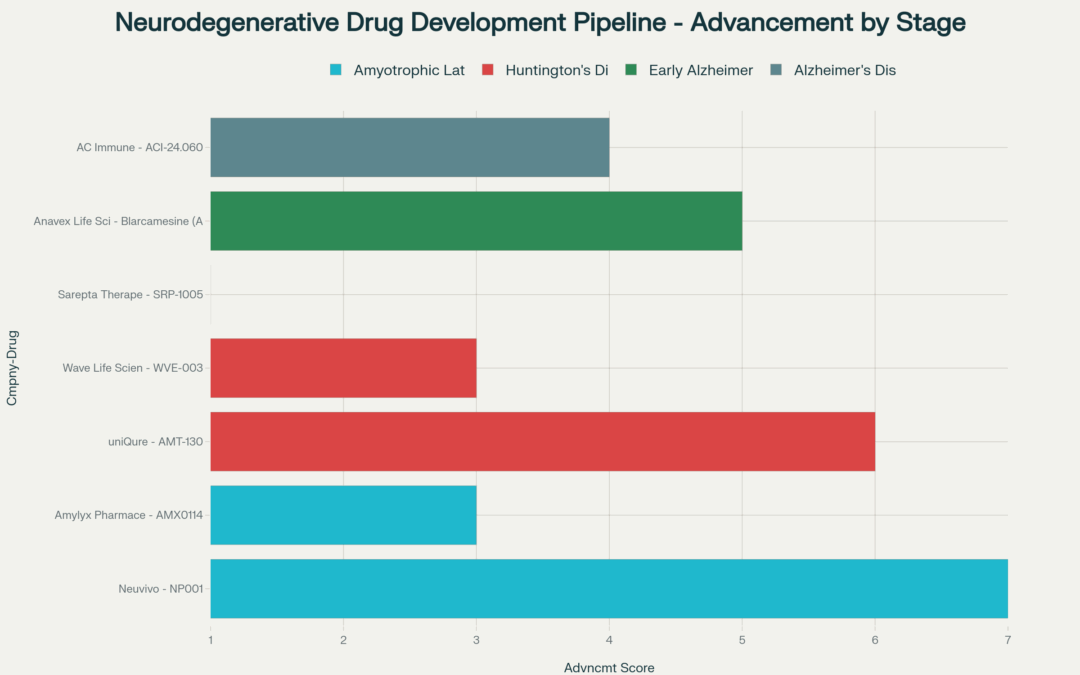
Among small and midsized companies, three stand out for having the most advanced drug development programs targeting neurodegenerative diseases. Neuvivo leads with NP001 for ALS, having submitted its New Drug Application to the FDA in October 2024. The company has achieved FDA alignment on the approval pathway and expects a decision within the next year.
A little further behind, uniQure received FDA Breakthrough Therapy designation for AMT-130 in Huntington’s disease. The company announced FDA alignment on its statistical analysis plan and Chemistry, Manufacturing and Controls requirements, positioning it for a Biologics License Application submission in Q1 2026.
Anavex Life Sciences announced positive precision medicine results from its Phase 2b/3 trials for blarcamesine in early Alzheimer’s disease. Recent data presented at the 2025 Alzheimer’s Association International Conference showed blarcamesine-treated patients continue to accrue benefit through up to 4 years, as measured by the pre-specified clinical endpoints ADAS-Cog13 and ADCS-ADL, respectively.
Huntington’s Disease Sees Multiple Approaches
Huntington’s disease has attracted significant research investment with three different therapeutic approaches in development. These are gene-targeted therapies, small molecule therapies, and regenerative medicine. Wave Life Sciences’ WVE-003 represents a first-in-class, allele-selective oligonucleotide. The SELECT-HD clinical trial demonstrated the first-ever allele-selective reduction in CSF mHTT protein while preserving healthy protein. Wave expects to submit an IND application for a potentially registrational Phase 2/3 study in the second half of 2025.
Sarepta Therapeutics intends to file its Clinical Trial Application (CTA) for SRP-1005 for Huntington’s disease by the end of 2025, with a clinical trial expected to begin in the first half of 2026.
ALS Research Shows Promise
Amyotrophic lateral sclerosis research has gained momentum with multiple companies pursuing different mechanisms. Amylyx Pharmaceuticals received FDA Fast Track designation for AMX0114 in June 2025. This investigational antisense oligonucleotide targets knockdown of calpain-2. Under Fast Track designation, AMX0114 is eligible for more frequent FDA meetings and potential Priority Review.
Alzheimer’s Disease Pipeline Grows
AC Immune advances multiple programs targeting Alzheimer’s disease. The company expects its ACI-24.060 anti-Abeta active immunotherapy ABATE Phase 2 trial to reach 12 months of treatment in December 2025, with interim results expected early 2026.
Leaders Driving Progress
Several senior executives are driving these developments forward across the industry.
At Amylyx Pharmaceuticals (LinkedIn), Co-CEOs Joshua Cohen and Justin Klee co-founded the company in 2013. Chief Medical Officer Camille Bedrosian leads clinical development strategy.
Wave Life Sciences is led by President and CEO Paul Bolno, who has served since 2013. Chief Scientific Officer Erik Ingelsson joined in May 2024, bringing extensive drug development experience. Senior Vice President Andrew Strahs heads biometrics.
Sarepta Therapeutics clinical development is led by Executive Vice President Louise Rodino-Klapac , who heads R&D and serves as Chief Scientific Officer. Executive Director Arani Chanda focuses on regulatory strategy.
At Anavex Life Sciences, President and CEO Christopher Missling brings over 20 years of healthcare industry experience. Vice President Daniel Klamer handles business development and scientific strategy.
AC Immune CEO Andrea Pfeifer co-founded the company and leads its precision medicine approach to neurodegenerative diseases.
Neuvivo CEO Ari Azhir founded the company in 2021 to develop advanced treatments for ALS and other neurodegenerative diseases.
Timeline to Market
The most advanced programs could reach patients within 12-24 months. Neuvivo’s NP001 for ALS has already begun FDA review following NDA submission. uniQure expects to submit its BLA for AMT-130 in Q1 2026. Wave Life Sciences plans IND submission for WVE-003 in the second half of 2025.
These timelines represent the culmination of years of research and could bring the first new treatment options to patients facing devastating neurodegenerative diseases. The convergence of multiple therapeutic approaches across different disease areas suggests the field is entering a transformative period for patient care.
Transform Your Healthcare Business Development Strategy
The rapidly evolving neurodegenerative drug landscape presents unprecedented opportunities for companies seeking to accelerate research and improve patient outcomes. Foculus Marketing provides exceptional market insights to help open new doors to healthcare innovation.
Our mission is to improve connections between innovative drug companies and providers of technology and services who can accelerate research while improving safety and exceeding quality goals.
Get in touch with Foculus to discover how we are transforming business development with AI and modern automation platforms.
Ready to discover new opportunities in the neurodegenerative therapeutics space?
Contact Foculus Marketing today to learn how our cutting-edge insights can drive your next breakthrough partnership.